
Manufacturing Change Management/ Quality Performance
Improvement
What makes changing organizations,
systems and processes to improve performance so tricky?
Well - first of all - not every manager is a change agent. The skill set to "run the business" is vastly different from that needed to "change the business". As a matter of fact, skillful and experienced change agents are hard to find. Many steps required to improve the performance of an organization are actually counterintuitive, and only experience can lead companies successfully through these changes. The change process is also difficult because most of the participants cannot see the end state, and are therefore uncertain about different roles, behaviors, and evaluation criteria.
Change is also about disciplined setting of priorities - knowing what to focus on and what can or must wait. Too many change processes fail from over-ambition, or killing the seeds of change by changing too much simultaneously. There needs to be a logical and attainable progression, and the patience and courage to live with some sub-optimal processes while the most important wins are gained.
Finally, change happens ultimately on the shop floor, but begins at the top. Getting senior managers to develop and articulate a clear philosophy, which is detailed to guide decision-making within the organization, is a step that is frequently overlooked. Translating that philosophy into concrete ways of working - or "Rules" is the next step, before any procedures are changed. Even the process of senior managers getting together to discuss and agree on philosophy, and middle managers agreeing on rules are important steps to changing how an organization collectively thinks and acts.
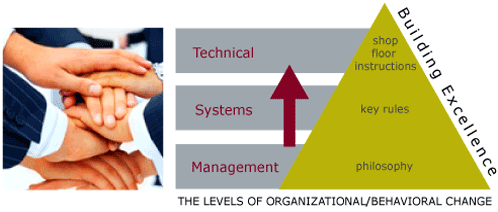
At BAI, we can design together with you a vision of where your organization wants to go - your goal, and can help to plan, step by step, how to get there. We can lead your organization through the change process, help to avoid the pitfalls, and build confidence and credibility that fuels the process. We are the best change agents in the business, and our references speak for themselves.
Data Integrity
BAI Partners have hands on practical experience in leading Data Integrity assessments and remediation efforts in direct response to regulatory inspections across laboratories, manufacturing and external manufacturing operations. Examples of BAI Data Integrity support includes:
-
Developing strategies supporting single site or global Data Integrity remediation.
-
Leading comprehensive investigation into the extent of the inaccuracies in data records and reporting.
-
Authoring and executing detailed investigation and assessment protocols to identify omissions, alterations, deletions, record destruction, non-contemporaneous record completion and basic DI principles (Attributable, Legible, Contemporaneous, Original and Accurate [ALCOA]).
-
Detailed assessments of chromatography data systems (CDS) including electronic records, supporting audit trails and user roles.
-
Developing custom Data Integrity training programs and communications to deliver basic DI concepts as well as cultural changes required to sustain compliant operations.
-
Conducting risk assessment using Quality Risk Management (QRM) methodologies of the potential effects of the observed failures on the quality of drug products and ongoing operations.
-
Developing corrective action plans that to ensure the reliability and completeness of generated data.
-
Conduct comprehensive root causes analysis of data integrity failures to fully understand motives contributing to the DI failure (Individual mistake, system errors, Individual malfeasance and Institution Malfeasance).
-
Develop interim measures that can be taken to protect patients and to ensure the quality of your drugs.
-
Long-term measures describing any remediation efforts and enhancements to procedures, processes, methods, controls, systems, management oversight, and human resources (for example, training, staffing improvements) designed to ensure the integrity of your company’s data.
cGMP/Regulatory Compliance and Quality Effectiveness Assessment and Remediation
For organizations that are planning to market products in the US and Europe, we can help in the regulatory compliance strategy preparation. We are highly effective in assessing manufacturing and testing sites for FDA compliance, and, unlike most corporate GMP auditors, we can quickly identify the "show stopper" issues and propose strategies for closingcompliance gaps in a pragmatic way that supports quality improvement within the business.
FDA Inspection Preparation and Follow-upCollectively, we have participated in, prepared for, and led more than 50 FDA
inspections. We've encountered a broad range of inspectors in a wide variety of
situations. We believe that having strong, effective, and transparent systems is
only a part of the equation when it comes to preparation for inspection -
getting people comfortable to present themselves credibly, and having an entire
manufacturing and quality team be able to seamlessly "sell" their organization
and operation is critical to success. - No "dog and pony shows" here, but just
thorough preparation under simulated "real life" inspection conditions - We can
offer strategies how to present critical issues, and can coach participants on
personal behaviors and effective communication.
Post Inspection - the inspection
isn't over until responses have been prepared and filed, and until they have
been accepted by the FDA. We can help assess and understand inspection findings,
work with the site to correct issues identified, prepare responses, and if
necessary help to continue a dialog with the Agency until all issues are
resolved.
BAI Partners have extensive experience in leading global system development, validation and deployment efforts. Some recent project examples include:
- Regional SAP design and implementation
- Design, building and testing of global Electronic Batch Record Systems (EBR), Laboratory Information Management Systems (LIMS) and Data Analysis Tools (DAT)
- Design, building and testing of global Regulatory Product Information Systems
- Requirements definition (AS IS and TO BE) for global systems to automate the Annual Product Review process
- Definition of requirements and technical architect for enterprise wide product information management systems
- Design, building and testing of integration middleware (Enterprise Service Bus) to facilitate the flow of product information from multiple enterprise authoritative data sources
When does cGMP stop being added value and start
becoming added cost?
Many organizations experience a lop-sided
emphasis on cGMP compliance, without the respective overall quality improvement
that is expected to accompany following the law. Too often the output ceases to
become high quality products and instead becomes just a mountain of useless
paper. The key is to balance cGMP compliance with good business practice, and
realizing that the two goals are not mutually exclusive.
It is possible to be
compliant to the regulations, and to have sleek and pragmatic quality systems
that make doing business easier and improve product quality. Let us show you!
Specialty
Programs:
Aseptic/Sterile Processing Guidance
With our in-house microbiologist and aseptic processing expert, as well as several partners who have managed quality operations for sterile manufacturing sites world-wide, BAI is uniquely suited to help address a wide variety of issues regarding manufacture, validation, and control of sterile products manufacture. From review of sterile facilities design through design of environmental monitoring and media fill programs, failure investigation, GMP assessment, and equipment qualification and process validation expertise, BAI can fill you sterile processing support needs.
Visual Inspection Programs and Parenteral Product AssessmentBAI can evaluate existing programs or develop new Visible Particulate Matter/Defect Inspection and Testing programs in parenteral facilities. Our specialty is to assist organizations new to visual inspection in fast tracking the basic inspection program development by providing essential background, training and guidance in a condensed time period. Our evaluations include reviewing/discussing/planning the following:
- Visual Inspection Practices/Procedures in Manufacturing
- Visual Inspection Practices in QA
- Inspection Booth Specifications and Vendor Selection
- Visual Inspection Test Standards for Particulates
- Visual Inspection Test Standards for Container/Closure Defects
- Visual Inspector Training & Qualification in Manufacturing and QA
- AQL Sampling Plans
- Acceptance Criteria & Referee Strategy
- Documentation Requirements (SOPs, etc.)
- Component Level Inspection and Preparation
- Customer Complaint Monitoring and Feed-Back
- Automated System Qualification
BAI has an experienced Microscopist on staff and can assist facilities with source determination of particulate matter problems or contamination events such as glass occurrences in finished product vials. Other focus areas where we can assist you in controlling particles are:
- Establishing a program of monitoring Intrinsic or Extrinsic Particulate Sources relating to products
- Systematic Particle Classification/Identification for Product Profiling
- Determination of washing, processing or filling equipment particulate contribution to the final product
- Assistance in establishing an internal Investigative Microscopy Laboratory
Validation Programs
BAI Partners have extensive experience with all aspects of process and laboratory validation technology including computerized systems and CFR part 11 compliance. BAI will take a team approach in developing the appropriate documentation from Validation Plans and User Requirements to IQ, OQ, PQ protocols. We can also assist you with specialized validation requirements such as:
- Stability Chambers and Walk-in Room qualification, Uniformity Testing to assure compliance to ICH Guidelines
-
Validation of Environmental Parameter Monitoring (temperature, humidity, differential pressure, etc.) or Data Logging Systems
- Qualification of Laboratory or Manufacturing Glass Washers
- Validation of Manufacturing and Packaging Systems
Laboratory Excellence Programs
BAI Partners lead Laboratory Excellence (LE) programs across small and large global organizations. Business benefits include: productivity gains of 20-60%, lead time reduction, Right First Time (RFT) quality improvements, compliance improvements and a more balanced and rewarding work environment for laboratory analyst. Laboratories that implement LE concepts yield trained individuals that become future leaders within organizations.
Laboratory Excellence modules include: mindset & behavior transformations, change management, lean concepts (leveling, flow, standard work, 5S), shift huddles, flexible workforce, reduced testing, method improvement, visual management, performance management and Gemba walks. Laboratory Excellence is further enhanced with paperless laboratory excellence modules including: Full paperless business and laboratory workflows, automation, instrument integration, enhanced analytical technology, e-visual management, QC testing at line and electronic performance management (KPIs).