The Highlands
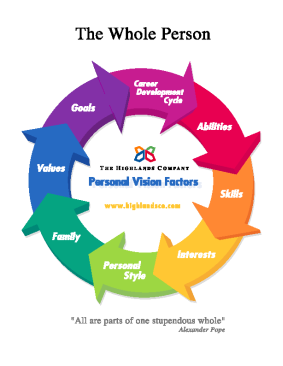
The Highlands Company is the publisher and sole distributor of the Highlands Ability Battery, an objective assessment of individual abilities. The Highlands Company strongly believes that people are happiest when they live and work by using their strongest abilities. To do this most effectively, they need to know and understand their abilities, as well as the other seven factors which combine to make the whole person.
The Highlands Company offers the Battery through a network of qualified and Certified Affiliates.
Our Managing Partner Margit Schwalbe-Fehl is certified to administer the Highlands Ability Battery and interpret the results for individuals and teams. The Highlands program is also extremely powerful to guide individuals through times of change and turning points in their professional careers.
Margit is also a certified Management and Career coach who is capable to develop and deliver tailored development programs that help individuals and teams to understand and apply their strengths in their professional environment, thus increasing career satisfaction and making individuals and teams more effective. We invite you to contact Margit (schwalbe-fehl@baipharma.com) to discuss possible applications for you personally and your team.
For more information on The Highlands Ability Battery: www.highlandsco.com